Arnold J. Levine is Professor Emeritus at the Institute for Advanced Study and leads the Institute’s Simons Center for Systems Biology in the School of Natural Sciences. An acclaimed leader in cancer research, he is also an expert on influenza and has advised the Canadian government and private industry on response to COVID-19. He spoke with Joanne Lipman, the Institute’s Peretsman Scully Distinguished Journalism Fellow, about the novel coronavirus outbreak, how it compares to previous pandemics, and potential therapies in the works that may help stop the spread. This interview was conducted on March 27, 2020. It has been edited for length and clarity.
Joanne Lipman: Your research spans a century of viral outbreaks, from the 1918 Spanish Flu pandemic to the present. From your perspective, how does COVID-19 compare to the 1918 Flu?
Arnold Levine: The 1918–1920 flu was the last influenza pandemic that really swept the world, and it was effectively lethal to 50 million people. The world population has grown ten-fold since 1918, so that 50 million today would be 500 million. But we are a lot more knowledgeable today with drugs, vaccine development, and information, so I hope we will never get to the scale of the 1918 flu pandemic.
JL: Is the new coronavirus similar to the 1918 influenza virus?
AL: The flu from 1918 really was a different virus completely than coronavirus. But these viruses do have in common that the pandemic strains of flu and the coronavirus both came from animals and both are respiratory viruses in humans. They do have in common that pneumonia plays an important role in the pathology.
The 1918 influenza, as all influenzas, was a bit of a smarter virus—if I could use the term—in that every year, the flu virus mutates a little bit. It has a very high mutation rate, a change in the nucleotides that make up the virus’ chromosomes. In that way flu escapes the immune system’s attempts to kill it.
Influenza viruses are unusual because we can become infected by a similar virus almost every year during our lifetime and occasionally there are worldwide pandemics that can cause many fatalities. Why does our usually excellent immune system fail us? How does this come about?
Arnold Levine explores these questions in a 2007 public lecture, "Tracking Influenza Virus Epidemics." Watch now.
Influenza has a really bad predilection of jumping from animals to humans. This also is true with coronavirus. Some viruses may live in an animal for many years, literally 100 years, and a whole generation or two of humans may have passed by without ever seeing that virus. In that case, there’s no immunity in the whole world’s population. But in the case of the 1918 influenza epidemic, the virus jumped from animals to humans just as COVID-19 did. The 1918 flu came out of chickens or some birds. COVID-19 came out of bats.
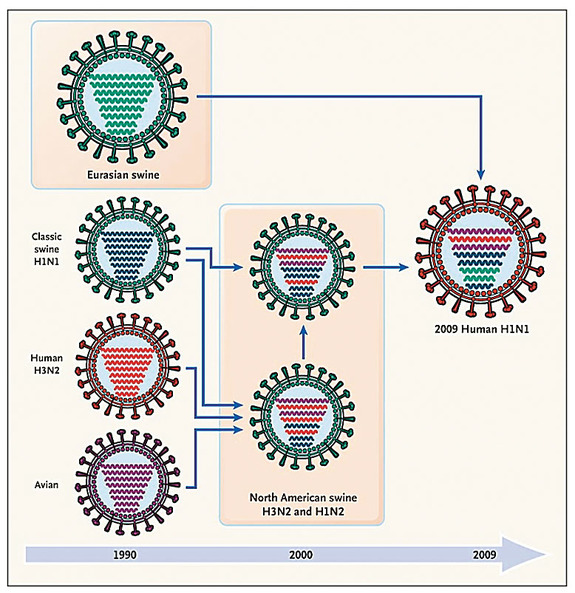
With the 1918–1920 flu epidemic—we could figure this out because of work we did at the Institute—seven of the virus’ eight chromosomes were chicken chromosomes and one chromosome came from the human flu. That virus was completely new—except for one chromosome—in the human population.
To give you a further point of comparison, influenza was and is a seasonal virus, it comes in the winter. In the spring of 1918 when that pandemic began, it was pretty mild. The second year, winter, was pretty vicious.
JL: Is it possible that COVID-19 could also become worse next year?
AL: We don’t know if next year will be worse. It depends on the number of people infected. Remember, in New York, of the people they tested, about 1 in 1,000 have been infected. That’s not many. There could be lots of susceptible people next year. The estimate of the number of people infected, however, is very poor. We do not have enough testing and some people, unclear how many, have mild cases of COVID-19 infection and may well become immune.
JL: COVID-19 has been compared to two previous viral epidemics, SARS and MERS. Is it more lethal, or less?
AL: The SARS virus, which also is a coronavirus, is very closely related to COVID-19. Like COVID-19, the SARS virus jumped from bats into humans in China. SARS had a nine percent mortality rate, pretty disastrous. As a point of comparison, present-day flu is 0.1 percent mortality. COVID-19 is somewhere between 1 percent to 2 or 3 percent. We haven’t got enough numbers yet. It’s hard to calculate those numbers at the beginning of epidemics.
But SARS had one thing that was very different from COVID-19. It only spread after people showed symptoms, when you coughed and you were very sick, when you literally went to the hospital. So, of the people who got infected with SARS and died, it was mostly the hospital workers and patients. It didn’t spread in the greater population. That’s the reason SARS stopped in a few years.
Now, in 2012, another coronavirus jumped from camels to humans—that was MERS. MERS is a coronavirus that comes from the Middle East—the acronym is Middle Eastern Respiratory Syndrome. It had a horrendous mortality rate, 50 percent. It never got out of the Middle East. By 2014—just two years later—it stopped. It had such a high mortality rate that people were dying, and you don’t transmit after you’re dead. It requires active infection to transmit.
JL: Why did COVID-19 become a pandemic, when SARS and MERS did not?
AL: COVID-19 takes less virus to infect us, and it has a long latent period with no symptoms so it can spread in a population. Also, unlike flu, coronaviruses can combine with one another, inserting foreign genetic information into its chromosome and changing the properties of the virus. We learn all that at the molecular level, from sequencing the chromosome of the COVID-19 first done in China, and testing its attachment to lung cells in a petri dish.
The other thing is, COVID-19 seems to have affected people in very different ways. One person can have the virus and not have many symptoms at all. Another person gets sick and dies.
The good news is that COVID-19 doesn’t mutate very much. It only has a single chromosome. So, there are fewer combinations, and no segregation of chromosomes.
What’s bad is, it settles in the lungs as pneumonia. The immune system attacks the virus-infected cells and kills your lung cells, the epithelial cells of the lining of the tubes to the lungs, and the lungs themselves. Then the lungs fill up with fluids, and that’s a pneumonia. Your oxygen intake is compromised, and eventually one could die from that.
So, this virus has been successful in becoming a pandemic because it spreads very efficiently and small amounts of virus can start an infection. That was not true of epidemic flu, SARS or MERS.
JL: You met with the Canadian government as part of a group of multidisciplinary scientists that identified three potential COVID-19 therapies. Can you share the three therapies with us?
AL: First, I learned at that meeting that there are eight vaccines in various stages of development. Some were actually in clinical trials. So, there’s a very big effort on vaccines going on right now by eight or ten different groups.
Second, there’s a big effort looking for chemicals that inhibit the virus. One of the genes of COVID-19 is an enzyme called a protease. When the gene was sequenced, it looked a bit like a human immunodeficiency virus protease gene—the HIV gene, that causes AIDS—for which we have a drug. So, that drug has been tried. And it does inhibit the coronavirus enzyme, but not as well as you’d like. So, better inhibitors are being made right now. Maybe there could be a pill that would stop the virus from replicating and stop the pathology.
The third potential therapy comes from an observation made in cancer treatment. There are certain cancer therapies that target a tumor, without killing healthy cells and that therapy may be able to be repurposed for COVID-19.
So, some existing approved cancer drugs are being repurposed. Now there’s a clinical trial going on in New York State, of 750 patients, with antibodies that we use for preventing autoimmunity in cancer therapy. It might be repurposed for COVID-19. I actually have high hopes for that one.
So to summarize, there are three approaches in therapy here that are important. First is vaccines. Second, there are these drugs that inhibit the virus. Then the third is an existing cancer therapy/drug that may be repurposed.
JL: One of the major roadblocks in the U.S. has been testing—both lack of availability, and length of time it takes to get results. Will we see improvements in both going forward?
AL: There are two ways to test for this virus. One way is to test for the virus directly. But there’s a better way, actually, to test the population at large, the whole population. That is not to look for the virus, but to look for antibodies. If you have been exposed to the virus and it replicates you make antibodies against it. We can test for that. You could prick your finger and get a drop of blood.
JL: Why are we not doing that now?
AL: Abbott just developed a home test that the FDA has just approved, for testing your blood.
JL: Can I get that test yet?
AL: No, they just got approval, and cleared the test for use in doctors’ offices and clinics.
JL: Looking ahead, how long will the current pandemic last?
AL: I think we’ll be intellectually and physically in better shape next year. We’ll see. The biggest problem is a lot of unknowns here. We don’t know anything really deeply about this virus.
But the good news is that it should not last forever. We need to be prepared however. There will always be a “next” virus.